Investigating a new therapy to help patients living with Stiff Person Syndrome (SPS)
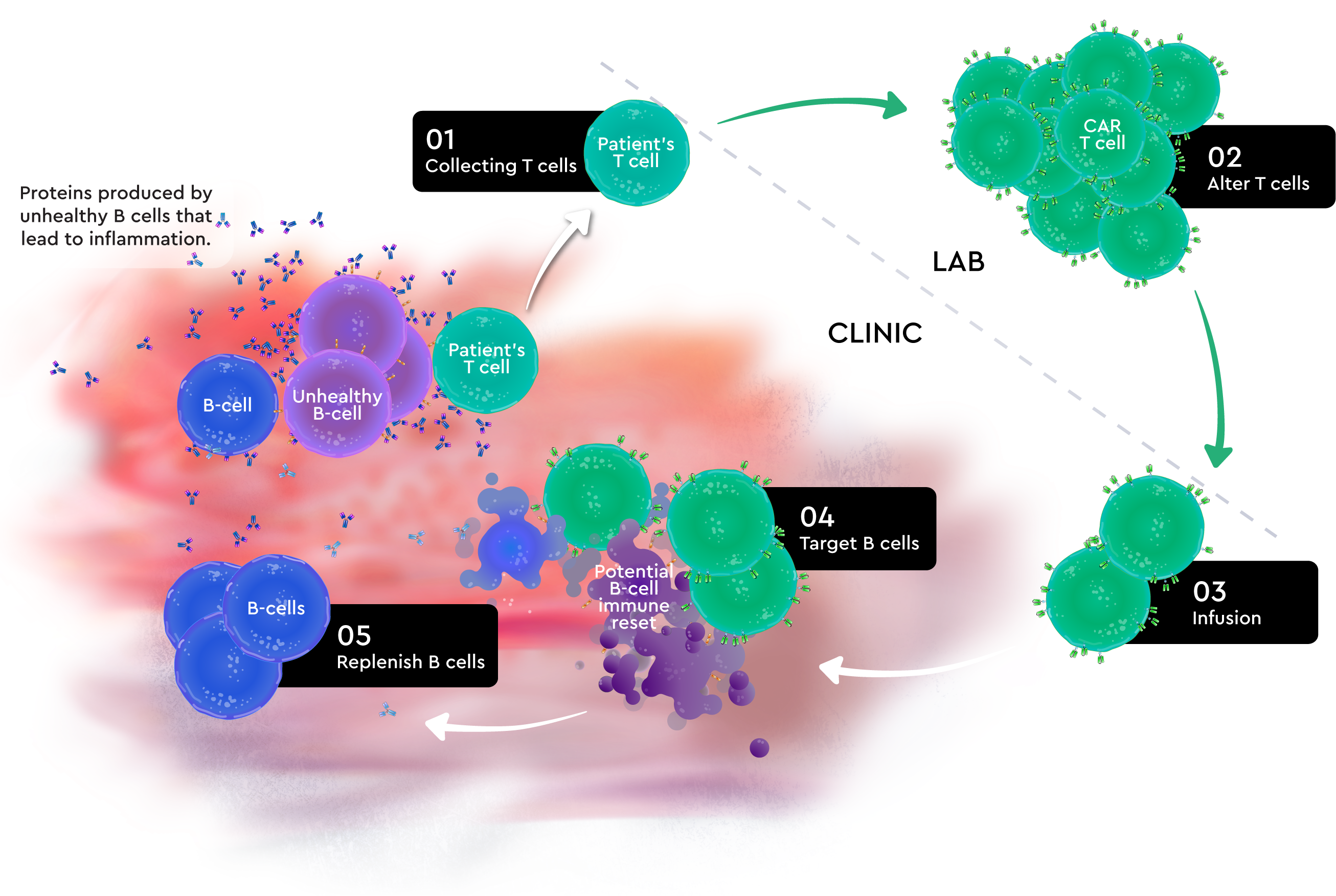
Contact UsKYV-101 is an investigational CAR T-cell therapy used in over 50 patients. KYV-101 works with your immune system to target the cells that harm your body, including unhealthy B cells that may contribute to disease activity in SPS.
This is the first study using KYV-101 in SPS,
but 3 patients with SPS have been treated with KYV-101. Patients with Lupus Nephritis,
Myasthenia Gravis, Systemic Sclerosis (Scleroderma), and Multiple Sclerosis
have also been treated with KYV-101.
This list does not include all the trial participation criteria and additional criteria may apply.
If you are interested in taking part in the trial,
your doctor can refer you to a trial site. The trial doctor will assess you to confirm your eligibility for trial participation. This may include performing additional tests. The trial doctor will
also explain the detailed criteria for participating. This trial has multiple
sites in the United States. You may need to travel if you do not live near a
trial site.
Participant Accommodations: We aim to
make every participant’s KYSA-8 clinical trial experience as easy as possible.
Accommodations for trial participation may be available and may include travel
and caregiver support.
If you are selected to participate, one of the doctors running the trial
(or someone on their behalf) will talk with you about what you can expect as a
participant in the trial so that you understand and are comfortable with what
it means to take part. This is called informed consent.
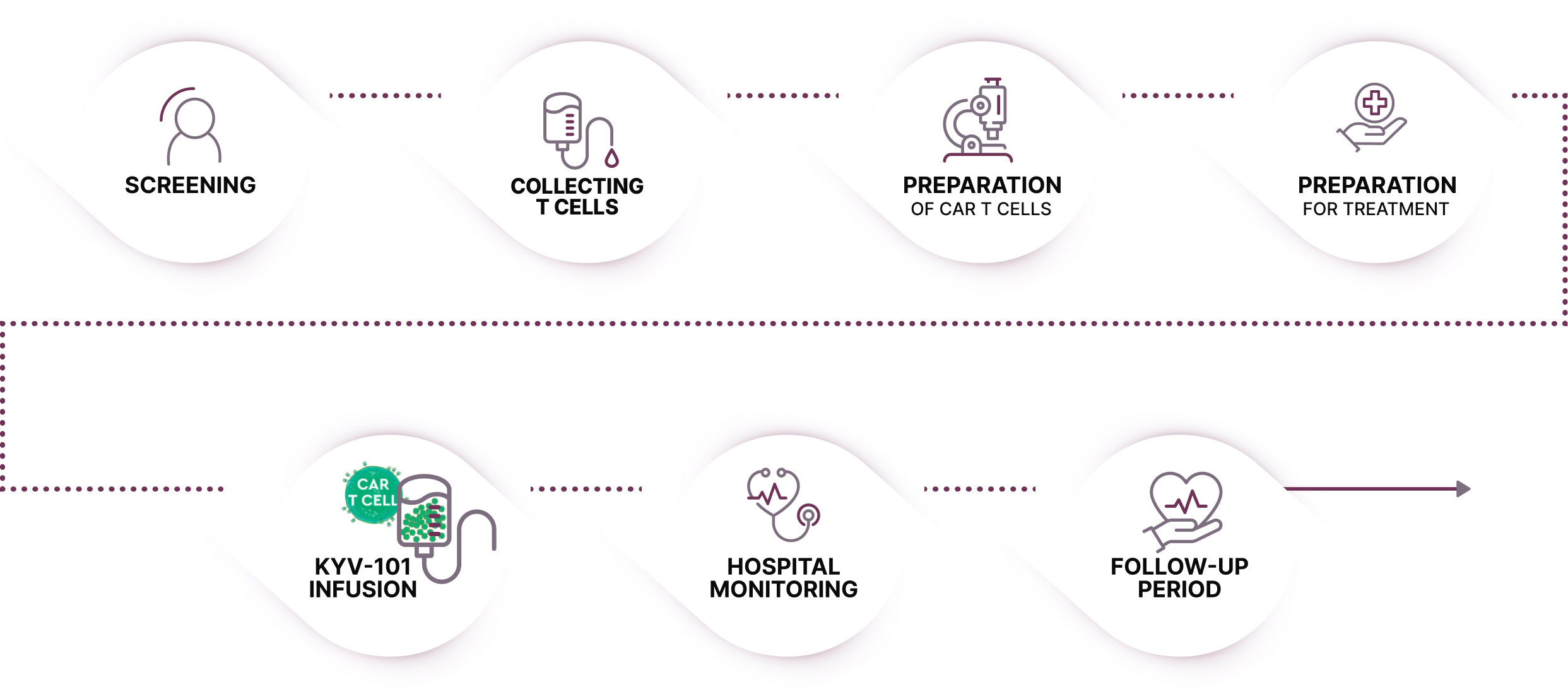
If you are able to enroll in the trial, these are the key steps you will be involved in:
CAR T-cell therapies can be associated with cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), which may be potentially serious or life-threatening but generally resolve within the first month after treatment.
People receiving this treatment are always in the care of a doctor, who monitors them closely for these side effects and can treat their symptoms to help prevent their worsening.